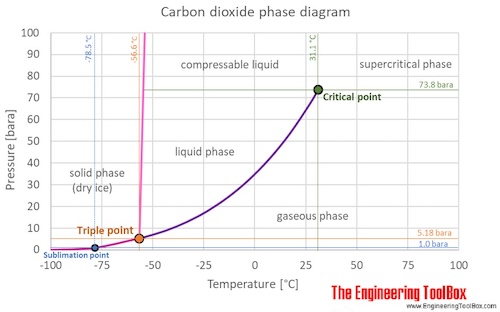
At 1 atmosphere near mean sea level pressure the gas deposits directly to a solid at temperatures below 78 5 c 109 3 f.
Carbon dioxide state at room temperature.
The symmetry of carbon dioxide and equal pulling of oxygen on either side of the carbon results in little to no intermolecular existing within carbon dioxide other than london dispersion forces.
75 psi under 31 1 c temperature of critical point and above 56 6 c temperature of triple point.
At 1 atm it is a solid at temperatures below 78 c.
To convert heat values to joules per mole values multiply by 44 095 g mol.
Dry ice is the solid form of carbon dioxide.
Carbon dioxide has no liquid state at pressures below 5 1 standard atmospheres 520 kpa.
In its solid state carbon dioxide is commonly called dry ice.
An aqueous solution turns litmus from blue to pink.
Msds for solid carbon dioxide is available from pacific dry ice inc.
Carbon dioxide co2 cid 280 structure chemical names physical and chemical properties classification patents literature biological activities safety.
A small part of the correspondence is due to the relationship between temperature and the solubility of carbon dioxide in the surface ocean but the majority of the correspondence is consistent with a feedback between carbon dioxide and climate.
Answered september 15 2016 at room temperature carbon dioxide co2 is a colorless odorless faintly acidic tasting non flammable gas.
Liquid carbon dioxide is the liquid state of carbon dioxide which cannot occur under atmospheric pressure.
When the carbon dioxide concentration goes down temperature goes down.
Dry ice will sublime or turn from a solid state into a gas state at room temperature.
If you left dry ice.
Structure and properties.
Co 2 is an acidic oxide.
194 7 k and the solid sublimes directly to a gas above 78 5 c.
The answer to your question is in the molecular structure of co2 there are 4 electrons in outer orbit of carbon and six in that of oxygen.
Carbon dioxide at room temperature is a gas.